Some science actually lies behind the tooth decay ads
Here’s how it works. The outer enamel layer of our teeth is the hardest tissue in the body. It is made of a mineral called hydroxyapatite that contains calcium and phosphate. Our saliva is mainly water but also contains calcium and phosphate.
There is normally a balance between tooth minerals and the minerals in saliva, close to neutral on the pH scale at 6-7.
When this drops below 5.5, calcium and phosphate molecules move out of the teeth and into saliva. They can pass the opposite way as part of teeth repair.
This demineralisation creates tiny pores in the tooth mineral and the enamel starts to dissolve. Initially, the pores are microscopic and can still be plugged by putting calcium or phosphate back in via our own saliva, or by replacing calcium with fluoride (this is how fluoride in toothpaste works to protect teeth).
However once the amount of lost tooth mineral reaches a certain level, the pores can no longer be plugged and the tooth tissue is lost for good.
The lady in the white coat on tv might have actually made good sense after all.


Acid in our drinks
Everyone knows sugary drinks are bad for our teeth and overall health. Their first port of call is our mouths where they come smashing in with their low pH and start attacking our enamel defence layer. Out rock the vital minerals that protect and strengthen our teeth, and unless we replace these (good foods, fluoride toothpaste), we risk decay getting a hold.
Coke comes in with a seriously low pH of around 2.6 – 2.7, just a touch less acidic than lemon juice and a shade more than old mate wine (3) and regular varieties of orange juice (3.5). Sparkling water’s carbonic acid rating is around 3.5, tap water is 7.
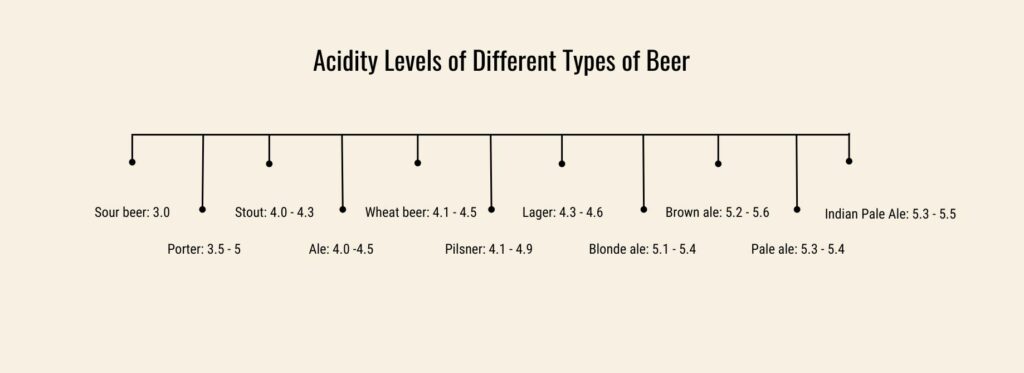
Beer (generally) has about the same acidity as whisky (around 4) and it’s more acidic than tequila (solid 5) but less acidic than brandies, (pH ~3.5). Stouts and sour beers can be as low as 3, while pale ales can be up to 5.5.